The immune system is the body's defense against infectious organisms and other invaders The immune system is made up of a network of cells, tissues, and organs that work together to protect the body,it is also help the infection of any pathogens
CST supports your immunology research with the highest quality antibodies. Immunology-based research focuses on the components of the human immune system and the possibility of their use to combat various diseases.
You need tools to identify and analyze immune cells and immune signaling to advance your research. CST is proud to produce top of the line antibodies for investigating proteins within the complex
signaling pathways of the immune system and to contribute to the international movement to develop better, more efficacious therapeutics.
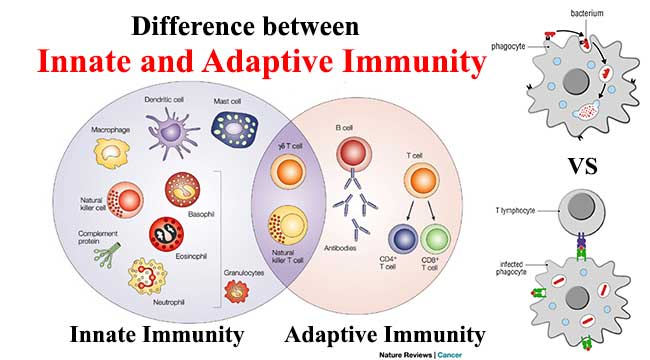
The immune system is comprised of two broad cellular responses:
- Innate immunity
- Adaptive immunity
The innate immune response is your first line of defense against pathogens. It provides a quick response to pathogens by many mechanisms, including cytokine production and complement activation.
The cell types involved in the innate immune response are phagocytic cells: neutrophils, macrophages, natural killer cells, basophils, and others.
As a leader in antibody development, CST has developed a wide array of highly effective antibodies against proteins involved in innate immunity such as the
STING,
NFκB, and
inflammasome signaling pathways
Adaptive Immunity
The adaptive immune response uses antigen-specific receptors to detect foreign antigens. This is a slow occurrence that results from efforts of T Cells, B cells, and natural killer T Cells. Humoral immunity uses antibodies for detection, whereas cell-mediated immunity uses T Cells to destroy the affected cells.
When the antigen is encountered for the first time, lymphocytes exert the primary immune response. The same cells can “learn” from their experience, so that a subsequent encounter with the same antigen will result in a quicker, secondary immune response.
CST has developed a wide array of highly effective antibodies to label the many proteins involved in the lymphocyte signaling pathways, including the
TCR and
BCR.
Immune Cell Signaling
The regulation of immune cells occurs through a number of key signaling pathways. Each pathway is comprised of a complex network of proteins that interact with one another to induce a specific cellular response to stimuli.
In addition to the STING, NFƘB, inflammasome, TCR, and BCR signaling pathways, the
JAK/STATand
TLR signaling pathways also play major roles in immune cell signaling
Development of T Lymphocytes
T Cells play a critical role in cell-mediated immunity and arise from lymphoid progenitor cells that originally developed from hematopoietic stem cells in the bone marrow.
T Cells are identified by the expression of CD3 and develop into their various subtypes in the thymus (hence the name T Cell). Each subtype is defined by the specific receptors expressed on the cell surface, making these cells highly selective to non-self pathogens. Mature T Cells are released into the bloodstream where they can be induced to become one of several classes of T cells including:
- Helper T Cell - CD4+ T Cells suited to recognize peptide antigens bound to class II MHC proteins and release a variety of cytokines.
- Cytotoxic T Cell - CD8+ cells that recognize virus-infected cells or tumor cells.
- Regulatory T Cell - The main job of regulatory T Cells (Tregs) is to maintain tolerance to self-antigens, as well as limit T-effector cell function and proliferation.
- Natural Killer T Cell - NK cells release small granules containing granzymes and perforin, which form pores and break down intracellular proteins in order to induce apoptosis in virally-infected or tumor cells.
T Cell Activation & T Cell Receptor (TCR) Signaling
T Cells become activated when a pathogenic antigen binds their cell-surface receptors. Each cell-surface receptor has affinity to a unique antigen, making T Cells highly specialized in antigen recognition.
Once the T Cell receptors bind an antigen, the T Cell will activate a series of
internal signaling pathways that allow for the antigen recognition to be verified. Only then will the T cell proliferate, expanding the pool of available cells that are specific for the harmful antigen, such as to different bacteria and parasites.
CST has a wide array of expertly validated antibodies that can distinguish T cell populations accurately and reproducibly by WB, IF/IHC, IP and extracellular/intracellular flow cytometry.
Using specific antibodies, individual subtypes of T Cells can be distinguished from a heterogeneous pool of immune cells. This type of immune cell subtyping is known as immunophenotyping.
Immunophenotyping of T Cells can be accomplished because T Cells have specific cell surface receptors, intracellular cytokine expression profiles, and/or differential phosphorylation of other intercellular proteins. This allow for selective labeling of these cells with specific markers. T Cells can first be immunophenotyped using IHC methods based on their CD3 expression, then they can be further sub-classified based on other well-characterized markers.
Development of B Lymphocytes
B Cells differentiate from hematopoietic cells found in the bone marrow. Immunoglobulin (Ig) receptors are assembled on the surface of B Cells and allow for specific recognition of a single antigen. Varying gene rearrangements during development lead to the differential expression of Ig surface receptors. Mature B Cells migrate to the lymph nodes and spleen after which they can further become memory B Cells or plasma cells.
While T Cells and B Cells are both able to recognize pathogens such as bacteria, viruses, and apoptotic cells, there two cell types are distinct in their characteristics and functions. B Cells develop in the bone marrow while T Cells are formed in the thymus.
The main difference is that T Cells can only recognize viral antigens outside the infected cells whereas B Cells can recognize the surface antigens of bacteria and viruses, which in turn results in antibody production.
B Lymphocyte Activation and Antibody Production
The critical cells that mediate antibody production are B Cells. Specific B cell receptors recognize and facilitate processing of antigens. B Cell activation occurs with help from T Cells, allowing the B Cells to mature into plasma cells that secrete antibodies.
The B Cell receptor (BCR) binds antigens, resulting in activation of multiple signaling cascades that involve kinases, GTPases, and transcription factors. This results in changes in cell metabolism, gene expression, and cytoskeletal organization. The complexity of BCR signaling permits many distinct outcomes including survival, apoptosis, proliferation, or differentiation into plasma cells or memory B Cells.
CST has a wide array of expertly validated antibodies that can distinguish B cell populations accurately and reproducibly by WB, IF/IHC, IP and extracellular/intracellular flow cytometry.
CST is your one-stop shop for B Cell immunophenotyping. CST has a wide array of expertly validated antibodies that can distinguish B Cell populations accurately and reproducibly by IHC including: